Nejsum Lab Research Focus
Our research is dedicated to unraveling the intricacies of epithelial function in both normal physiological states and pathophysiological conditions.
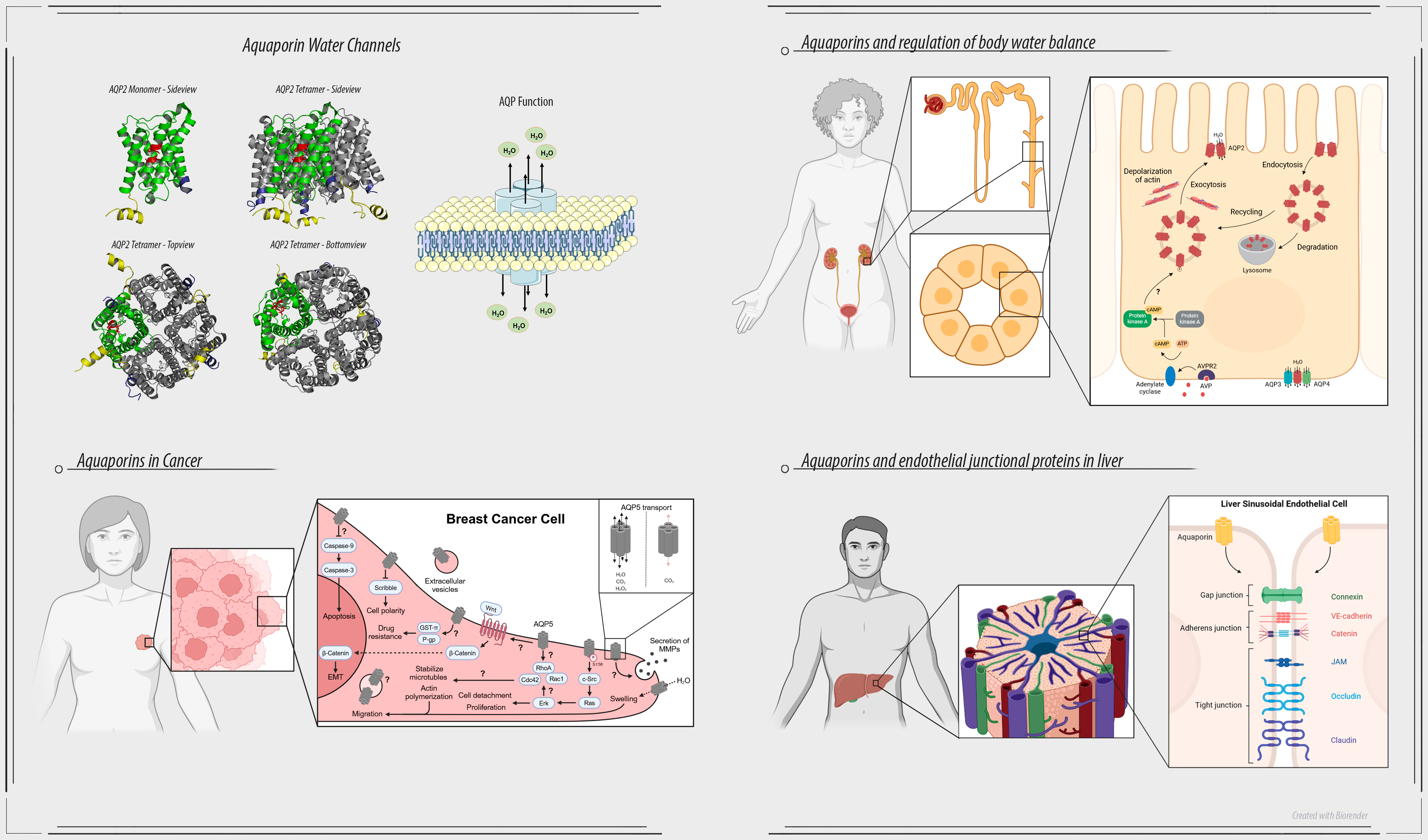
A central focus is aquaporin (AQP) water channels, which are pivotal in maintaining body water homeostasis and implicated in cancer development.
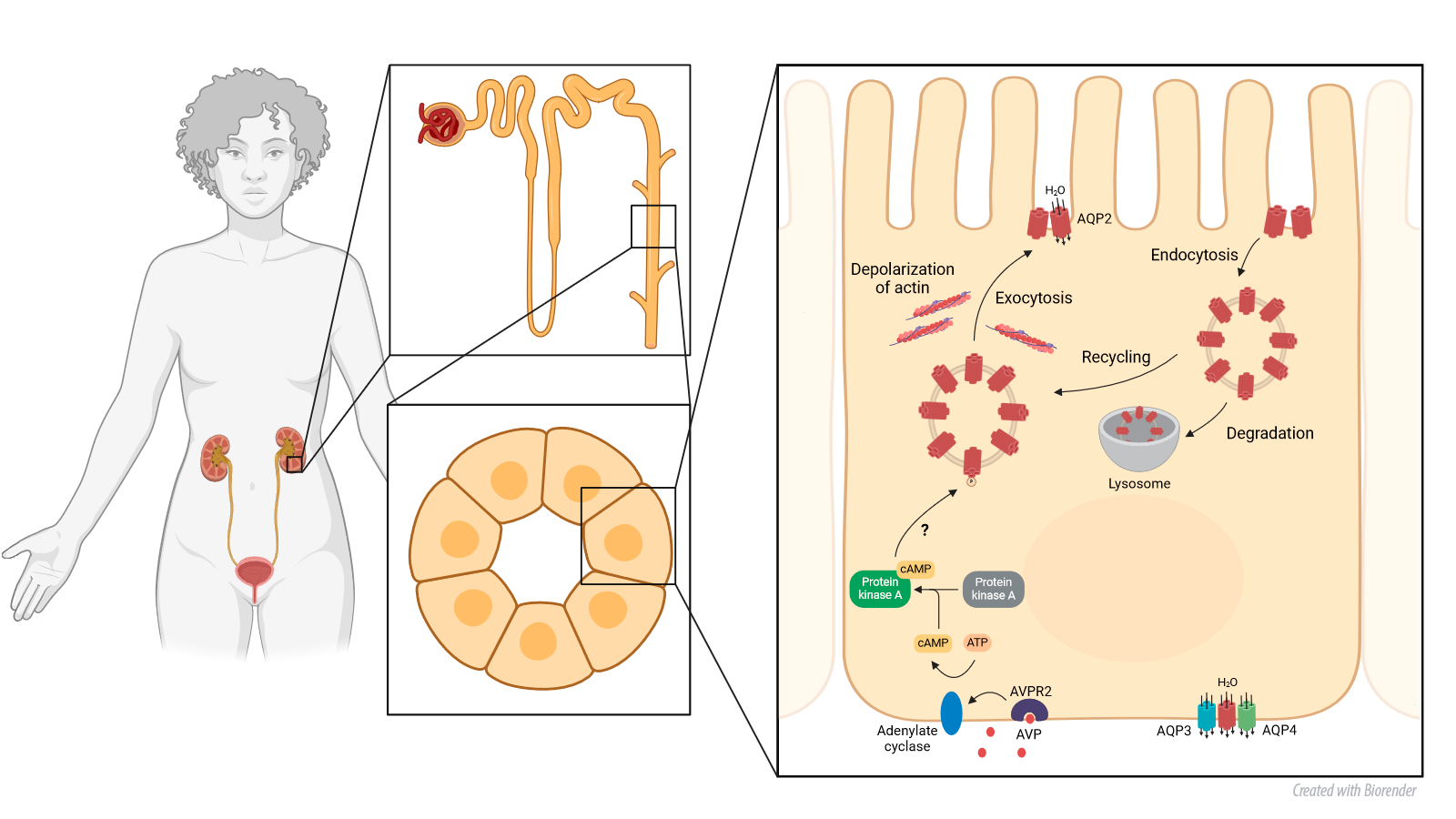
The laboratory is investigating the AQP2 vesicle population in renal collecting duct principal cells. AQP2 is pivotal in regulation of body water homeostasis and is implicated in various diseases associated with body water dysregulation. Utilizing cutting-edge super-resolution microscopy at the Advanced Imaging Center, Janelia Research Campus, USA, we have achieved the first ever visualization of the three-dimensional AQP2 vesicular network. Our extensive analysis has led to the proposal of a modified model for the mobilization of AQP2 vesicles in response to hormone stimulation. Furthermore, the implementation of state-of-the-art expansion microscopy, coupled with advanced image analysis techniques within our laboratory, has provided unprecedented insights into the vesicle population.
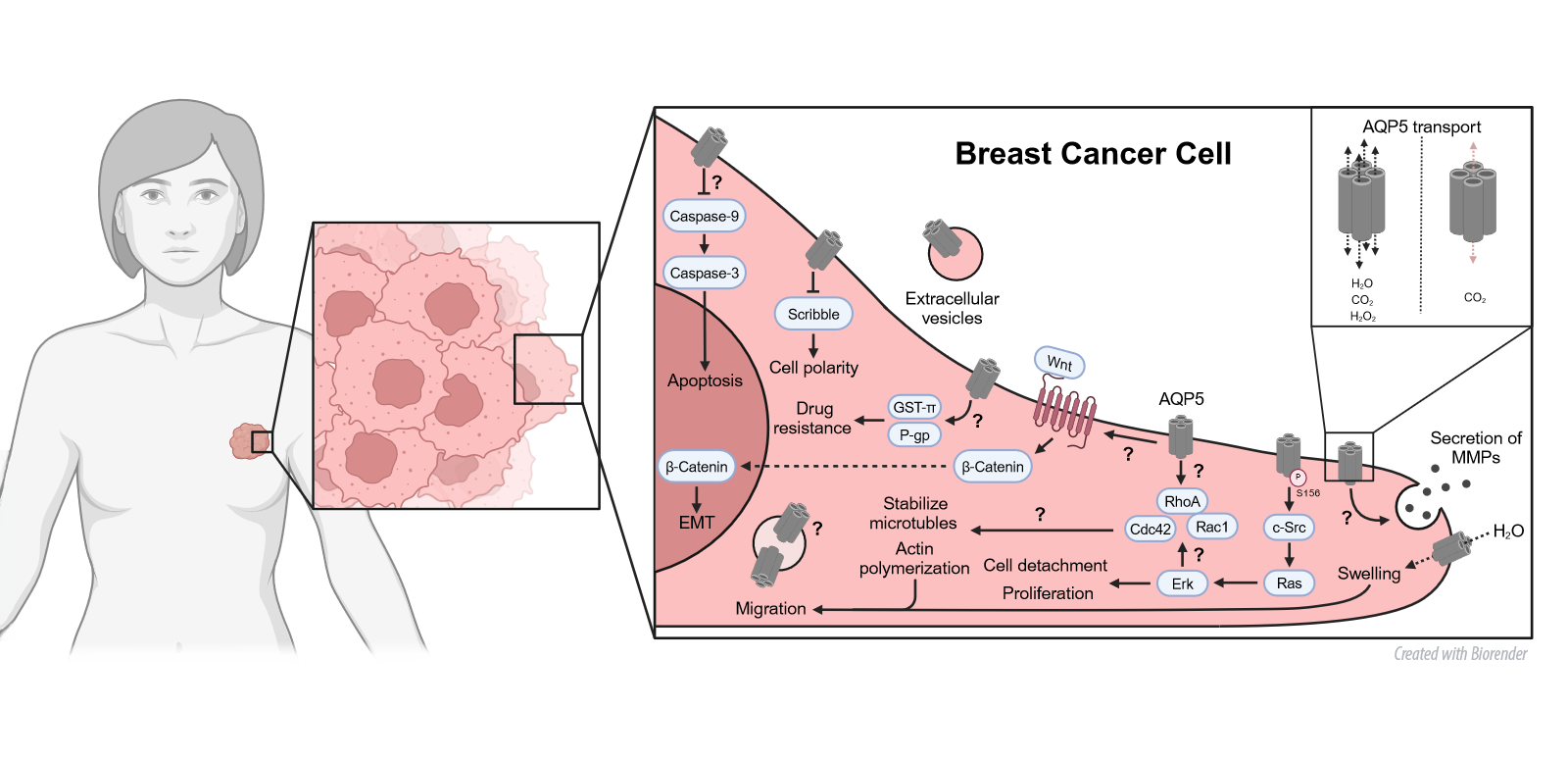
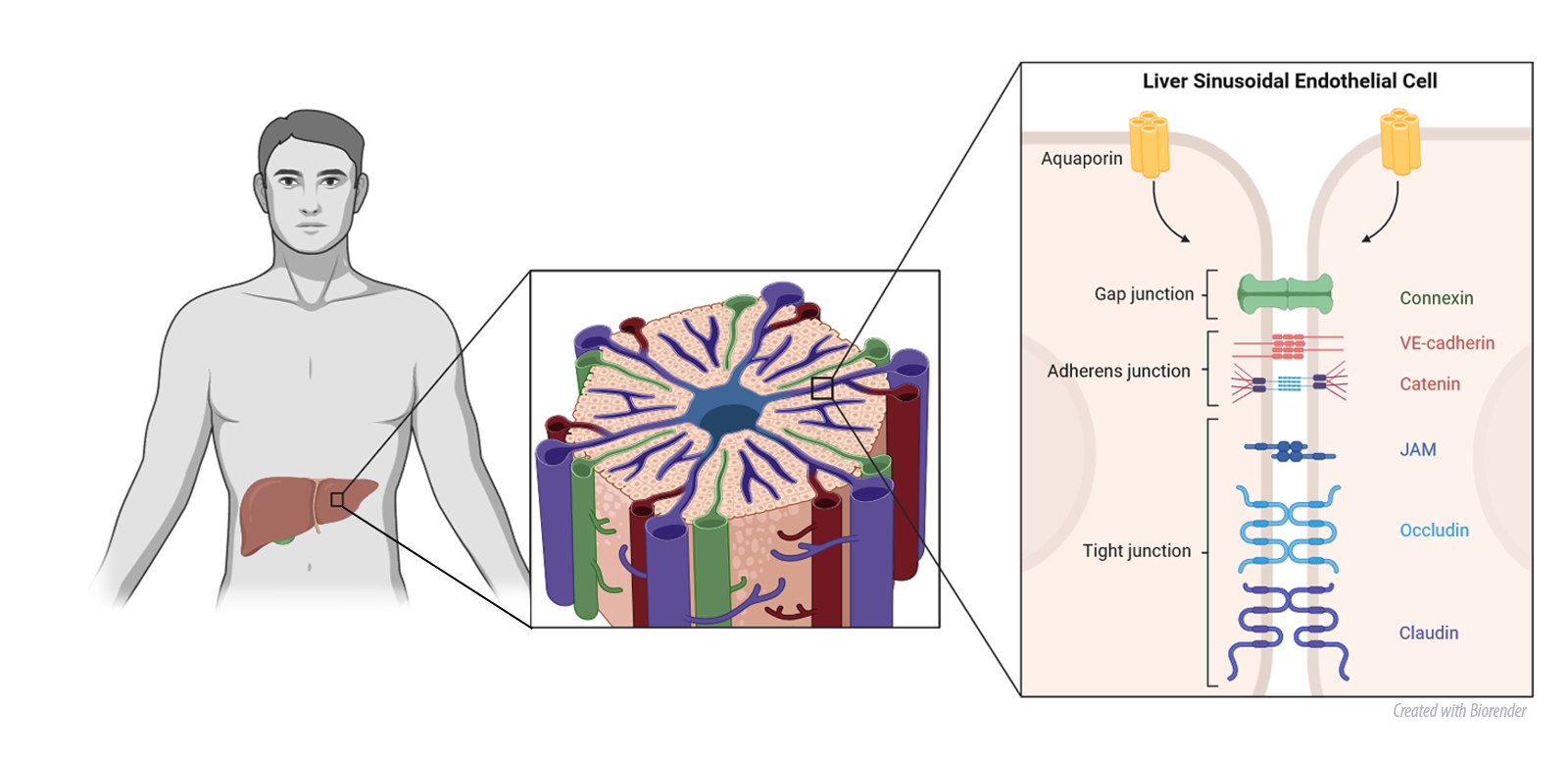
Another aspect of our research are novel facets of AQP functions, elucidating their distinctive regulatory roles in cellular morphology, adhesion, collective cell migration and cellular polarity—all pivotal mechanisms in cancer development. We have shown that AQPs differential regulate the response of 3D breast cancer spheroids to anticancer therapy. These discoveries not only advances our understanding of cancer development but also points toward potential therapeutic interventions and personalized treatment strategies. Building on these findings, we also study how AQP expression is altered in liver diseases and how this affects junctional proteins of especially the microvasculature.